INTRODUCTION
Skeletal metastasis, synonymous with metastatic bone disease (MBD), is the term used to describe the clinical scenario of visceral cancer metastasizing to bone. Approximately 1.8 million new cases of cancer arise per year in the United States (US), and about 15% of all carcinomas manifest clinically as bone metastases.1 The most common sites of origin are lung, breast, kidney, thyroid, and prostate.2–4 These lesions can lead to pathological fracture, hypercalcemia, spinal cord compression, and debilitating pain.4,5
The role of the orthopaedic surgeon in the multidisciplinary care of patients with skeletal metastases should not be overlooked. Survival after diagnosis of metastatic carcinoma varies based on site of origin, along with other patient- and disease-specific prognostic factors.6–8 There have been significant advances in systemic treatment, translating to improved patient survival within the last decade.9–11 The use of these biological and targeted therapies often leads to a discordant response, with continued bony progression despite improvements in the visceral burden of the disease. This phenomenon has led to patients living longer but requiring ongoing orthopedic treatment for palliation of their progressive MBD.9 Appropriate orthopedic surgical interventions allow patients with MBD to maintain both quality of life and functionality.9,12 This report aims to review the evaluation and treatment of lower extremity skeletal metastases for the non-oncologic orthopedic surgeon. Specifically, we aim to:
-
Provide an overview of the diagnostic approach to MBD, including imaging, evaluation of impending pathologic fractures, and prediction of patient survival.
-
Provide an overview of treatment approaches, including non-surgical and surgical options.
-
Introduce decision-making philosophies for non-oncologic orthopedic surgeons to consider palliation, function, and the multidisciplinary aspects of cancer care.
DIAGNOSIS AND EVALUATION
Presentation and Workup
The most common presenting symptom for patients with skeletal metastases is increasing bone pain that is not resolved with pain medication or other analgesic measures.12,13 Metastatic carcinoma causes pain due to (1) impending pathologic fracture and resultant bone/periosteal distortion with weight-bearing, (2) completed pathologic fracture, (3) tumor compression on neurovascular structures, and (4) increased intraosseous pressure caused by marrow-based disease.13 Symptoms are often confused with age-related degenerative joint disease or spine conditions, injury, overuse, etc. Performing a thorough history and physical examination, evaluating diagnostic studies, and considering a patient’s disease progression are critical in distinguishing between cancer-related pain and pain from other causes.
Imaging of skeletal metastases should start with plain radiographs.14,15 Computed tomography (CT) is preferred for visualizing cortical integrity as well as juxta-articular lesions.13 Magnetic resonance imaging (MRI) is used to evaluate soft tissue masses, visualize bone marrow replacement, and depict neurovascular involvement.14
Besides comprehensive history, exam, and imaging, the gold standard for diagnosis of MBD remains tissue biopsy.13,16 For patients who have not had a previous biopsy of a bony lesion, it cannot be assumed that a patient with a history of carcinoma and a new bone lesion has metastatic disease of the suspected carcinoma; a second primary diagnosis must also be ruled out. Once one bone lesion is confirmed to be metastatic carcinoma, subsequent bone lesions can be assumed to represent the same histology. The five most common carcinomas to metastasize to bone are listed in Table 1.
Treatment Considerations
Once a metastatic lesion is detected, three treatment options exist including observation, palliative radiation, or surgical intervention.28 Extent of symptoms and risk of pathologic fracture are the two key determinants of treatment, along with consideration of more global patient factors. A tool that is useful in this treatment determination is the Mirels scoring system.29–32 This criteria aims to predict the risk of pathologic fracture based on four characteristics of skeletal metastasis: size, lytic/blastic character, anatomic location, and severity of pain. The calculated score, which represents the lesion’s risk of pathologic fracture, can range from 4 to 12, with the indication for surgical intervention being a total score ≥ 8 [Table 2].33 Since this scoring system’s inception, additional factors have been found to influence the risk of fracture, including advanced age, a visual analog scale (VAS) score >6, administration of antiresorptive agents, functional status, tumor histology and anticipated response to radiotherapy, etc.30 Quantitative CT-based structural rigidity analysis and finite element analysis are associated with improved positive and negative predictive value, sensitivity, and specificity in predicting fracture risk, compared to applying Mirels criteria alone.31,32,34 Unfortunately, the availability of this technology is limited. Therefore, the authors recommend applying the Mirels scoring system as an initial step in decision-making. Consideration of the patient’s functional status and life expectancy is also critical, as indicating them for surgical or nonsurgical treatment should align with their overall prognosis. Time spent recovering from treatment should always be less than their expected survival.
Survival Estimation and Its Relation to Orthopedic Decision-Making
As predicting life expectancy is notoriously challenging in these patients,35,36 PATHFx was developed as a clinical decision-support tool based on machine learning to improve this prediction.37–40 It is particularly useful to orthopedic surgeons when deciding if surgery– and which surgery– may be indicated for a patient with metastatic carcinoma. When using PATHFx to assess surgical candidacy, the goals of surgical treatment should be palliation of pain, immediate postoperative weight bearing, and preservation of independence and functionality; these objectives should be achieved by the intervention being considered long before the patient is likely to die of their disease.9,41 Furthermore, the durability of their orthopedic construct should outlast the patient’s life expectancy to minimize the need for retreatment or revision surgery. An understanding of the risks of local progression of disease, implant failure, and other complications should be assessed on a case-specific basis by the surgeon. For example, tumor resection and endoprosthetic reconstruction of a solitary femoral renal cell metastasis may be preferred over intramedullary fixation in a patient whose PATHFx-predicted survival is several years.
TREATMENT
Non-Surgical Treatment
A brief overview of the pathophysiology of skeletal metastases is needed to understand non-surgical treatment options. When disseminated carcinoma cells create a metastasis in bone, they secrete parathyroid hormone-related protein (PTHrP), which stimulates the release of receptor activator of nuclear factor kappa beta ligand (RANK-L) from osteoblasts. This substance activates osteoclasts to initiate bone resorption.42–46 This process continues until an osteolytic lesion forms, compromising the integrity of the bone. In the development of sclerotic bone metastases, endothelin-1 is secreted by the tumor, which leads to osteoblast-driven bony deposition.
It is standard of care for all patients with MBD to receive antiresorptive therapy, which targets the process described above. These agents include denosumab and bisphosphonates. Denosumab decreases bone resorption by inhibiting RANK-L-mediated osteoclastic activation.47 Nitrogen-containing bisphosphonates inhibit farnesyl pyrophosphate synthase, which is responsible for osteoclast attachment.48 Non-nitrogen bisphosphonates form analogs of adenosine triphosphate and cause apoptosis of osteoclasts.49 These drugs decrease the frequency of skeletal-related events by up to 38%,50-52 which include pathological fracture, spinal cord compression, pain or instability requiring surgical intervention, re-irradiation, and hypercalcemia.5,50–52 The most common adverse effects of antiresorptives are nausea and vomiting (20-30% of users are affected), with the rarest but most devastating being osteonecrosis of the jaw and atypical femur fracture.53–55 Preventative measures such as good oral hygiene and mindfulness of treatment duration can mitigate these risks.54
In conjunction with antiresorptive therapy, radiotherapy plays a critical role in the management of skeletal metastases. It can be administered alone as a palliative strategy or in the adjuvant setting after surgery.52 Palliative radiotherapy, aimed at alleviating pain but not curing the patient of their disease,56,57 has been shown effective in 50-80% of patients with MBD when indicated appropriately.5,52 Patients with symptoms who do not meet the criteria for prophylactic surgery based on Mirels Criteria or other guidelines should be referred to a radiation oncologist for palliative treatment. Breast and prostate histologies will respond more favorably than lung or renal cell histologies.58,59 While the doses administered are usually low enough to avoid severe toxicity, the most common side effects of radiotherapy to the skeleton include overall fatigue, local dermatitis (80-90%),60 radiation fibrosis syndrome (30%),61 neuropathy, radiation osteitis, and pathologic fracture (1.2-25%).1,62–64
Surgical Treatment
Certain patients with symptomatic skeletal metastases will be candidates for surgical intervention based on the factors described above. The goals of surgical intervention should be to alleviate pain and improve/maintain physical independence and function. Rarely does orthopedic intervention for MBD directly affect prognosis or survival; however, it can be argued that preservation of physical function may secondarily mitigate the sarcopenia and failure to thrive that can occur in end-stage cases of metastatic carcinoma and very specific cases of limited renal cell metastatic disease a patient’s life can be prolonged by orthopedic intervention.65,66 Surgical techniques involved in treating impending or completed pathologic fractures vary widely and will be discussed based on anatomic location in the sections below. In almost all cases, to minimize disease progression and construct failure, postoperative radiation should be administered after wound healing.
Pelvis and Sacrum
The pelvis is the second most common site for bone metastases, often associated with pathologic fracture.67,68 The redundancy of the bony pelvis’ structure allows small bony defects to be well-tolerated. However, surgical treatment may be considered when these defects are substantial, pain limits ambulation and non-surgical treatments have failed.
Acetabulum
The traditional approach to treating periacetabular metastases has been the Harrington rod technique, first described in 1981.69 An acetabular implant is stabilized through a scaffold that is created using bone cement and multiple threaded pins passed from the ilium to the ischium and pubis [Figure 1].67,69 Historically, the Harrington procedure is associated with acceptable outcomes: one study showed prosthesis survival of 92% at one year and 89% at five years. Plaud et al. found that Musculoskeletal Tumor Society (MSTS) scores improved from an average of 31.1 pre-operatively to 67.7 at a six-month follow-up and 82.4 at a 12-month follow-up.70 This study found a reoperation-free survival rate of 76.1% at 6 and 12 months; the main complications were pin migration and infection. Houdek et al. found an all-cause reoperation rate of 27% in patients who had undergone Harrington-style reconstruction,71 and other studies demonstrate up to 25% risk specifically of local progression of periacetabular disease, which could lead to mechanical failure.72 With the acceptance of these complications and the historical limited life expectancy of patients with metastatic carcinoma, the Harrington reconstruction technique was the workhorse procedure to address periacetabular metastatic disease for several decades surgically.
Recent developments have introduced less-invasive procedures to address periacetabular metastatic disease associated with decreased perioperative morbidity/mortality compared to the Harrington technique.73,74 For example, ablation, osteoplasty, reinforcement, and internal fixation (AORIF), cementoplasty, and percutaneous screw stabilization have proved to be effective interventions for periacetabular metastases and fractures [Figure 2]. These can be outpatient procedures through small incisions, allowing for immediate weight-bearing and near-immediate re-initiation of systemic treatment that would otherwise need to be postponed in a larger open periacetabular reconstruction setting.73,74 Studies have reported up to 23% improvement in MSTS scores and a reduction in VAS pain scores by up to 7 points.73 Compared to open procedures, these operations have minimal postoperative hardware complications, fractures, infections, or wound complications.74 Local control of disease after AORIF may be unfavorable compared to wider tumor excisions and larger reconstructions; however, the value of these procedures cannot be underemphasized in patients with limited life expectancy (therefore a limited opportunity for progression of the disease to occur) and goals of immediate, low-risk palliation of pain.
Another alternative to the above techniques is the acetabular reconstruction strategy using highly porous tantalum acetabular components and augments. Houdek et al. describe this technique and showed an improvement in Harris Hip Scores from 37 to 72 (p<0.01) associated with this technique.75 The authors also reported a 35% rate of disease progression in their cohort of 37 patients (compared to 15% in the 78 patients undergoing Harrington reconstruction).75 However, only an 8% all-cause reoperation rate (0% for acetabular loosening specifically) was found, compared to the 27% rate found associated with the traditional Harrington technique, suggesting that these porous tantalum implants are relatively durable even in the setting of acetabular disease progression.71 While arthroplasty-trained specialists are more familiar with these implants, it is essential for orthopedic oncologists to familiarize themselves with these options, given their favorable durability and outcomes.
Occasionally, a patient will present with intractably painful periacetabular metastases without viable reconstructive options. In this situation, resection arthroplasty can be considered.76 While a considerable leg length discrepancy results, this is a relatively straightforward and safe treatment for palliating painful pelvic metastases and preserving some level of independent ambulatory function while minimizing postoperative implant-related complications. While custom implants can also be considered to reconstruct large periacetabular defects, they are not favored given the significant economic and time cost associated with their creation, considerable complication rate, and overall goals of a patient with metastatic carcinoma.
Ilium and Pubis
The first line of treatment for skeletal metastases of the ilium and pubis is radiotherapy, as there is low mechanical stress in these areas.68 pathologic fractures of the iliac crest, anterior superior/inferior iliac spine, or rami often result from metastases in these locations; however, these fractures usually heal with conservative management and palliative radiation. Rarely is surgical treatment indicated for refractory lesions and is usually performed as an intralesional procedure or a minimal extralesional procedure without reconstruction, as these portions of the pelvis are expendable. In recent years, percutaneous screw fixation and AORIF have become increasingly used.
Sacrum
The sacrum is a common site of bone metastasis within the pelvis due to its vascularity and anatomic location. Treatment is indicated when the lesion causes sacral nerve root compression or loss of integrity of the spinopelvic weight-bearing axis, leading to weight-bearing pain.77 Palliative radiation is commonly relied upon. However, when biomechanical support is needed, minimally-invasive percutaneous bone cement injection, screw fixation, and AORIF are effective at palliating pain from sacroiliac insufficiency and improving independent ambulation.78
Femur
Proximal Femur
The proximal femur is the most common location for pathologic fracture in MBD.79 It is also a common location for surgeons to consider prophylactic fixation, given the propensity for metastases and the high stresses across this area. As explained in previous sections, consideration of Mirels criteria, PATHFx, and multiple patient factors can aid in decisions regarding prophylactic surgery. Multiple retrospective nonrandomized studies have shown that patients who undergo prophylactic fixation live longer and are associated with decreased healthcare costs compared to those who undergo fixation of a completed pathologic femur fracture.80–82 In a patient with a life expectancy longer than three months, prophylactic fixation of painful proximal femur lesions should usually be undertaken as long as perioperative risk is not prohibitive.
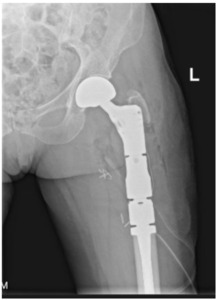
The surgeon must decide the appropriate procedure when a patient meets operative indications. The intervention must provide stability for the remainder of the patient’s life, minimize revision rate,82 and maximize time after recovery for them to enjoy their functional improvements. Surgical options include arthroplasty, intramedullary nailing, and plating.83 Any considerable metastatic disease in the femoral head indicates arthroplasty. Hemiarthroplasty versus total hip arthroplasty depends largely on the surgeon’s judgment regarding acetabular disease, underlying arthritic change, life expectancy, functional status, anticipated hip stability, and other factors. The conversion rate from hemiarthroplasty to total hip arthroplasty for acetabular wear in patients with metastatic disease is extremely low (1%), suggesting that the durability of hemiarthroplasty in these patients is more than acceptable in most circumstances [Figure 3a-d].84
When the metastasis exists in the basicervical femoral neck or peritrochanteric area, debate exists over whether internal fixation or endoprosthetic replacement is the most appropriate surgical option. While traditionally, the majority of these patients would be treated with intramedullary nailing, a study of 298 patients conducted at Memorial Sloan Kettering Cancer Center showed an incidence of IM nail failure of approximately 6.1% at an average 14.7 months, and plating was associated with a 42% failure rate.85 Mechanical failure from tumor progression and persistent loadbearing through the implant usually occurs between 12 and 15 months,88,89 and patient survival after surgery is the most important factor in predicting revision.86 It must be remembered that nails and plates were designed for non-pathologic traumatic fractures with reliable healing potential, which cannot be assumed with pathologic fractures. Catastrophic implant failure or painful disease progression often requires conversion to proximal femoral replacement, which undermines the goal of providing the patient with a durable, revision-free construct [Figure 4].
With these failure rates of internal fixation in mind, along with improved patient survival achieved in recent years, the utilization of arthroplasty implants is increasingly justified.3 If patient survival is anticipated to be greater than 6-12 months, the surgeon should strongly consider performing a durable endoprosthetic reconstruction– either hemiarthroplasty or proximal femoral replacement– over internal fixation. Traditionally, these implants are cemented due to periprosthetic fracture risk, unreliable biologic fixation of press-fit stems in pathologic bone, and radiation exposure; over the last few years, a few articles have called this into question and suggested similar success rates of press-fit and cemented stems.87,88 The complications associated with these arthroplasties occur earlier and differ from the mechanical failures seen with internal fixation.89 They include wound complications, periprosthetic infection, dislocation, and aseptic loosening.90 Cemented stems also introduce the risk of bone cement implantation syndrome, a poorly understood and fatal complication involving hypoxia, hypotension, and/or unexpected loss of consciousness occurring around the time of cementation or prosthesis insertion.91,92 If the patient survives the first several months without suffering one of these complications, the implant survival rate of prosthetic reconstruction is favorable overall compared to internal fixation strategies (3.1% revision rate reported by Steensma et al.).85 This advantage must be weighed against the prolonged operative time, higher risk of immediate surgical complications, increased cost, and prolonged postoperative recovery.
Diaphyseal femur
Impending or completed pathological fracture of the diaphyseal femur is commonly approached with intramedullary nailing with cement reinforcement [Figure 5].93 Pulmonary complications associated with reaming or cementation must be considered, and Cipriano et al. suggest using a reamer-irrigator-aspirator system to reduce tumor embolization and microemboli.94 Certain tumor histologies, such as renal cell carcinoma, are also notorious for local tumor progression around a nail; however, excellent durability is expected in most cases: Tanaka et al. showed 94% implant survival at three years with intramedullary nailing of femoral metastases.95 Although rarely used, intercalary prostheses are occasionally indicated for diaphyseal metastases in the primary or revision setting. They have been associated with fairly good functional and palliative outcomes with significant improvement in MSTS and VAS pain scores.96,97 Still, they are more technically challenging to implant and involve increased perioperative risk compared to standard intramedullary nailing.
Distal femur
Metastases to the distal femur are commonly addressed with internal fixation techniques such as locked plating with cement augmentation and retrograde intramedullary nailing. Internal fixation can be performed if the articular surface, subchondral bone, and majority of the cortex are intact.98 Seo et al. reported improved visual analog scale (VAS) pain scores from 8.1 preoperatively to 2.7 postoperatively one week after plating and cementation of distal femur pathologic fractures.99 When the burden of disease on the distal femur is considerable and compromises the distal femur articular surface, arthroplasty is a viable surgical option. Johnson et al. conducted a retrospective study with 15 patients who obtained tumor endoprostheses about the knee based on tumor pathology as well as the extent of bone loss. All patients had improvement in MSTS and Knee Society Scoring System (KSS) and significant pain reduction at the final follow-up, with a 13% reoperation rate.100
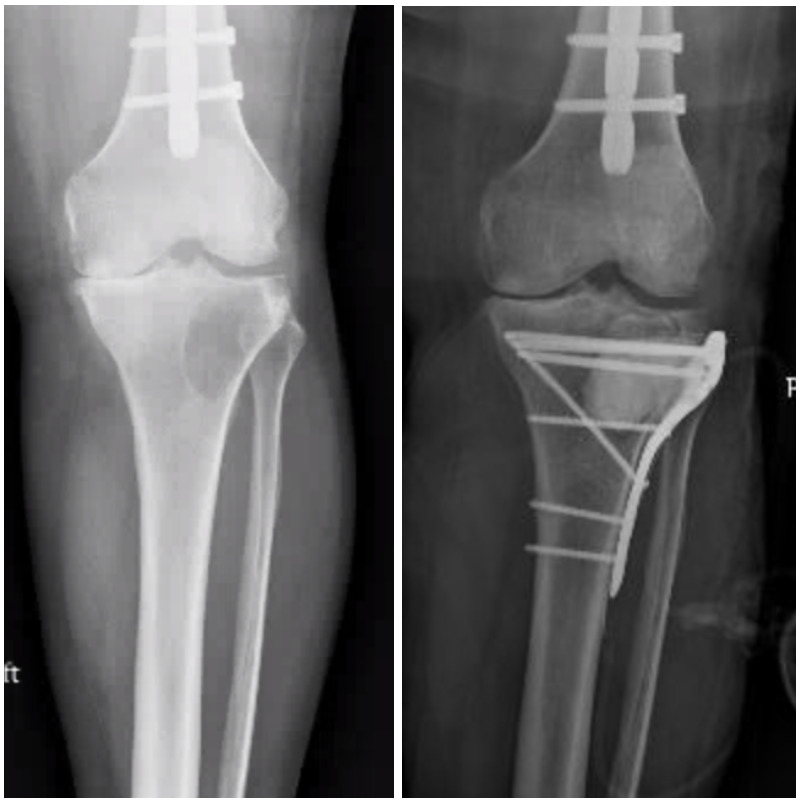
Tibia
Acral metastases, defined as lesions distal to the elbow and knee, are often encountered in cases of advanced metastatic carcinoma and tend to be, therefore, associated with limited patient survival.101 Sixty-eight percent of acral metastases are to the tibia, and the tibia is the third most common long bone overall to develop metastatic disease.102,103 Often, these lesions can be treated effectively with palliative radiation.2,104 Locked plating with cement augmentation is commonly used when surgery is indicated in periarticular locations such as the tibial plateau and plafond [Figure 6]. The subcutaneous nature of these anatomic locations introduces considerable wound complications and infection risk, which are reported at 12% by Bonnevialle et al. For skeletal metastases within the metadiaphyseal or diaphyseal tibia, intramedullary nailing provides relatively simple stabilization, achieving pain relief and restoring ambulatory function.104 Post-operative radiotherapy is critical to minimizing the local progression of disease. Due to primarily compression forces in the tibia as compared to tensile forces that exist in the proximal femur, mechanical failure of tibial constructs is a less frequent complication.101
Conclusion
The approach to managing pelvic and lower extremity skeletal metastases varies greatly based on tumor histology, lesion-specific characteristics, and patient-related factors. Interventions should align with the overall care goals, specifically achieving pain palliation, restoring immediate postoperative weight-bearing, and maximizing functional independence. Often, palliative radiation satisfactorily achieves these objectives. If surgery is indicated, the construct should durably outlast the patient’s anticipated life expectancy and be followed by postoperative radiation to minimize the local progression of the disease. In light of recent advances in systemic therapy, orthopedic surgeons should recognize the potential for prolonged survival in patients with metastatic disease and embrace a nuanced and patient-specific approach to treatment decision-making.
Declaration of conflict of interest
The authors do NOT have any potential conflicts of interest for this manuscript.’
Declaration of funding
The authors received NO financial support for the preparation, research, authorship, and publication of this manuscript.’
Declaration of ethical approval for study
No ethical approval is required for the review article.
Declaration of informed consent
There is no information (names, initials, hospital identification numbers, or photographs) in the submitted manuscript that can be used to identify patients.



._preoperative_and_postoperative_x-rays_of_two_cases_of_metastatic_bone_disease_to_th.png)




._preoperative_and_postoperative_x-rays_of_two_cases_of_metastatic_bone_disease_to_th.png)