INTRODUCTION
Cervical myelopathy is a common, debilitating condition affecting a significant portion of the aging population due to spondylosis.1 Patients with myelopathy may experience gait disturbances, paresthesias, difficulty with fine motor tasks, and other symptoms.1 Symptoms of myelopathy range from mild to severe and sometimes catastrophic. A narrative review of the natural history, pathophysiology, diagnosis, indications, and surgical management follows.
REVIEW
Cervical myelopathy (CM) is a common spinal cord disorder with a variable clinical presentation. This disorder in the elderly is characterized by degeneration of the cervical spine, leading to compression of the spinal cord and subsequent neurological dysfunction.2 The symptoms of CM are often characterized by insidious onset with periods of relative stability or quiescence punctuated by a stepwise decline in neurologic function.3 Clinical manifestations of this condition include neck pain and stiffness, spasticity, balance and gait dysfunction, impaired dexterity, dysesthesias in the upper and lower extremities, and bowel/bladder dysfunction.
Early and accurate diagnosis of cervical myelopathy is critical for effective management and prevention of neurological deterioration. Diagnosis begins with a comprehensive clinical history and physical exam. Imaging such as computerized tomography and magnetic resonance imaging are essential for diagnosing and surgical decision-making.4 Several surgical approaches have been described in the literature. Still, the choice of intervention is determined by several patient and surgeon factors, including the location and extent of cord compression, spinal alignment and stability, and surgeon training.
Pathophysiology
In cervical myelopathy, the structural changes to the spine often occur due to degenerative processes. Myelopathy may also result from more acute injury. The spondylosis begins with disc degeneration, resulting in relative segmental instability, loss of disc height and bulging, ligamentum flavum buckling and hypertrophy, and facet arthrosis. These changes cause narrowing of the canal and compression of the spinal cord. Compression of the spinal cord can be primarily anterior, posterior, or combined. Dynamically, the spinal cord has reduced mobility through stenotic segments, may be actively compressed during physiologic motion of the cervical spine, or may be compressed by abnormal spine motion, such as spondylolisthetic segments.2 Current understanding of the biological changes resulting in CM’s clinical manifestations is limited and borrows heavily from spinal cord injury studies. Chronic compression of the spinal cord may result in disruption of the microvasculature, leading to chronic ischemic injury to the cord.5 Similarly, the spinal cord–blood barrier disruption may result in secondary inflammatory changes or injury.6,7 Lastly, ischemic and inflammatory injuries may trigger further cell death and axonal loss via apoptotic pathways.5 Objectively, spinal stenosis is defined as an anterior-posterior canal diameter of less than 13 mm. The spinal cord is likely to be impinged at this size, generating clinical findings. Several other bony spinal canal measurements have been used to characterize the extent of stenosis. Still, more recent studies have found the volume-occupying rate of the spinal canal to be a more reliable way to assess stenosis radiographically. The volume-occupying rate of the spinal canal in a neutral position was higher in patients with CM than in normal subjects.8
Clinical Findings
The variable presentation of mild to extreme symptoms – often makes diagnosis challenging.9,10 Patients with CM have consistent, progressive symptoms instead of transient or intermittent symptoms.1 Common symptoms include neck pain and stiffness,11 spasticity, gait and balance dysfunction,9 impaired dexterity,9 dysesthesias in the upper and lower extremities,11 and late findings of bowel and bladder dysfunction.1 Symptoms can have a wide range of severity from mild changes to florid myelopathy, where patients may struggle to stand upright due to impaired balance stemming from compromise of the spinal tracts of the dorsal columns. A thorough physical examination is also key to identifying subtle findings of upper motor neuron and dorsal column dysfunction. A complete neurological exam should include an assessment of the patient’s gait, all major myotomes and dermatomes of the extremities, deep tendon reflexes, Romberg’s test, Hoffman’s sign, the inverted brachioradialis reflex, Babinski’s reflex, and presence of sustained clonus. These findings can range in severity similarly to the patient’s symptomatology and require a high index of suspicion to identify. Another consideration is the presence of superimposed radiculopathy, which may result in relative hyporeflexia.12 Once a clinical diagnosis of CM is considered, imaging should be obtained to corroborate clinical findings.1,9,11
Imaging
Radiographs
Plain radiographs are often the first imaging modality. Two orthogonal cervical spine views are typically sufficient (anterior-posterior and lateral). However, additional views such as obliques to evaluate the bony neuroforamen and dynamic flexion and extension views are useful to provide additional context. Flexion and extension views of the cervical spine are important tool for dynamic evaluation of spinal stability and can identify subtle bony translations that may influence surgical strategy. Plain radiography can readily identify spondylotic changes to the cervical spine, such as disc collapse, osteophyte formation, facet arthropathy, and alterations to spinal alignment. However, it has limited value in assessing the spinal canal where compression occurs. Alignment measurements can be performed on radiographs and may influence the surgical approach or technique.
Magnetic Resonance Imaging
MRI remains the gold standard for evaluation of CM.4 MRI provides a high-fidelity, multiplanar reconstruction of the bony spine, neural elements, and soft tissue envelope of the cervical spine. Different anatomic structures can be evaluated in detail through various acquisition sequences and image post-processing. MRI images provide the best method for identifying which spinal levels are stenotic, what structures contribute to the stenosis, and the severity of stenosis and/or cord compression.9 The maladaptive formation of disc-osteophyte complexes in the anterior canal, buckling and hypertrophy of the ligamentum flavum, and facet hypertrophy posteriorly are the predominant sources of stenosis. MRI can also identify subtle changes within the spinal cord parenchyma, such as T1 hypointensity and corresponding T2 or Short Tau Inversion Recovery (STIR) signal hyperintensity. This may correlate with myelomalacia or spinal cord edema, both late findings of spinal cord compression, which may represent irreversible changes to the spinal cord. Lastly, recent advances in MRI technology may be useful for prognostication. Diffusion tensor imaging (DTI) can evaluate the individual tracts within the cord parenchyma. As this technology develops, it may be able to differentiate patients who can be reasonably monitored versus those who would benefit from early surgical decompression. This is especially important, considering up to 61% percent of asymptomatic patients have signs of anterior cord compression.12 MRI is an invaluable tool in the diagnosis, prognosis, and surgical planning aspects of CM.
Computerized Tomography
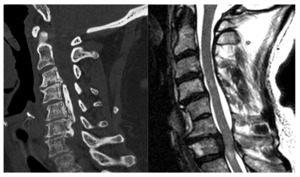
CT is also uniquely suited to the evaluation of CM. When MRI is contraindicated, CT myelography is a useful option to identify and quantify severity, though it lacks the prognostic benefits of evaluating the cord parenchyma. CT scanning is also paramount for positively identifying ossification of the posterior longitudinal ligament (OPLL), a clinical entity wherein the PLL ossifies along the dorsal vertebral body, resulting in canal stenosis and resultant neck pain, stiffness and neurological symptoms in some. OPLL can cause stenosis at the disc level as well as retro-vertebral. If this entity is not recognized preoperatively, it can lead to serious intraoperative complications.11 CT is also useful in pre-surgical planning as it better assesses bony anatomy and planned instrumentation. [Figure 1]
Classifications and Prognosis Factors
Grading systems of symptom severity have been developed, which are useful tools for classification, prognostication, and monitoring recovery after initiating treatment. The modified Japanese Orthopedic Association (mJOA) score is the most encountered system. The only modification from the original JOA score was to amend the use of hashi (chopsticks) while eating with a spoon.13 Patients are considered to have severe myelopathy with a score of less than 12 points and mild with a score of 15 or more points. Other systems include the Nurick and Ranawat grades, but these are less detailed and reflect progressive debilitation.14,15
Several studies have evaluated patients undergoing conservative care for CM to determine prognostic indicators for progressive disease. Two studies evaluated patients with “rapidly progressive” CM. However, the definition of rapid progression is variable. In one study, this was considered a progressive neurologic deficit over one month. Both studies identified the presence of T2 signal hyperintensity within the cord parenchyma as an independent risk factor for progression.16,17
Surgical Management
The indications for surgical intervention are reserved for patients with moderate to severe clinical symptoms or with progressive neurological decline. Earlier intervention in the process of decline is associated with better long-term functional outcomes.5 A variety of surgical approaches are available to decompress the cervical spine with unique advantages and risk profiles. Surgical interventions may be broadly categorized based on approach (anterior vs posterior) and fusion vs motion preservation (laminectomy decompression, laminoplasty, total disc arthroplasty). Several factors, including the location of the pathology, number of levels of compression, sagittal alignment, presence of OPLL, and surgeon preference/experience dictate the surgical approach.
Anterior Approaches: Anterior Cervical Discectomy and Fusion (ACDF), Anterior Corpectomy and Fusion (ACF), Cervical Disc Replacement (CDR)
Anterior Cervical Discectomy and Fusion (ACDF)
ACDF can be considered a minimally invasive procedure. It utilizes the intermuscular Smith-Robinson interval of the anterolateral neck. The disc material from the offending level or levels is removed, decompressing the ventral spinal cord. Bony osteophytes are concomitantly resected. Interbody grafts or implants are used to restore disc height, reconstruct cervical alignment, and produce additional indirect canal decompression by posteriorly re-tensioning collapsed ligamentum flavum. ACDF is also well suited to addressing concomitant neuroforaminal stenosis. The use of anterior plating for additional stability varies by region but is commonly performed in the United States.18 ACDF can safely decompress three or even four levels of stenosis. However, there are significant risks. Increased operative time and the addition of more cranial cervical levels increase the risk of postoperative dysphagia, injury to the recurrent laryngeal nerve, and life-threatening airway complications.18
Anterior Corpectomy and Fusion (ACF)
Anterior subtotal or total corpectomy and fusion can be performed through an anterior approach. This technique may be required for multilevel reconstructive procedures and to address retrovertebral stenosis, as in cases of OPLL or migrated disc herniations.19 A similar approach to the ACDF procedure is performed with discectomies above and below the levels of stenosis. The intervening body is then resected. The remaining posterior wall of the body can be resected completely or isolated and left to “float” on the ventral dura as a bone island. The latter may be necessary in cases of adherent OPLL where there is a high risk of massive durotomy with complete resection.20 The interbody can be placed with a structural allograft, titanium cage, or PEEK (PolyEtherEtherKetone) cage. An anterior plating system can stabilize short segment corpectomy, such as one body. Multilevel corpectomies should be stabilized in a “360 degree” fashion with staged or concomitant posterior arthrodesis/instrumentation.21
Cervical Disc Replacement (CDR)
The decompressive aspects of CDR are virtually the same, with special attention paid to the endplates to avoid subsidence of the CDR implant. In elderly myelopathic patients with advanced spondylosis (including facet joints), CDR is generally contraindicated.22 In young patients with large, soft disc herniations, which result in myelopathy, a CDR can be a good option23,24 compared with ACDF. CDR presents unique technical challenges, such as careful endplate preparation, implant positioning, and unique complications.25 Device designs include mobile bearings that may dislocate or wear over time.26 Implants may subside within the body or form extensive heterotopic ossification, which results in a de facto fusion.27,28 CDR in myelopathy remains somewhat controversial as there are theoretical risks of further microtrauma to the diseased spinal cord with motion preservation compared with immobilization and remodeling, which occurs in fusion surgery.24
Posterior Approaches
Laminectomy
Cervical laminectomy is an excellent method of decompression of predominantly posterior compression of the spinal cord or scenarios where an anterior approach may be challenging. A laminectomy removes the spinous process, lamina, and ligamentum flavum while preserving the facet joints laterally. A lordotic to the neutral spine will allow the spinal cord to “drift back” away from anterior structures. There is a risk of postoperative kyphosis due to violation of the adjacent posterior tension band.29 In patients with preoperative instability or kyphotic alignment, laminectomy alone is generally not favored.30
Posterior Cervical Decompression Fusion (PCDF)
PCDF can be considered when pathology is primarily posterior-based and with favorable alignment (non-kyphotic). Compared with laminectomy alone, the rate of complications with the addition of posterior fusion is minimal.30 Strong consideration should be given to the additional fusion construct in patients with questionable alignment, degenerative facets, and instability.30 Anterior pathology of OPLL and long constructs (>3 segments) also warrants posterior consideration to avoid complications of durotomy, dysphagia, and pseudoarthrosis. Fixation is accomplished via screw-rod constructs, with the cervical lamina, lateral masses, and pedicles being traditional fixation points. [Figure 2]
Laminoplasty (LP)
LP is a non-fusion procedure that preserves the posterior bony and ligamentous structures while expanding the canal. Two common techniques described are the “open-door” and “French door” techniques. The open-door technique is the earliest described technique from 1983 by Hirabayashi et al.31 A partial thickness trough is cut along the spinolaminar junction of the treated levels. A full-thickness trough is made along the opposite side. The full-thickness cut can then be opened against the “hinge” opposite, expanding the canal diameter. The opening can be held in place with bone blocks or other implants. The French door laminoplasty splits the lamina in the center. A partial thickness trough is made at the bilateral spinolaminar junction, allowing the two leaves of the lamina to be gently opened from the center. The opening is held in place with bone blocks or other implants. Similar to laminectomy, the LP procedure should be reserved for patients with neutral to lordotic alignment, allowing the spinal cord to “drift back.” Due to the mechanics of the posterior cervical spine, laminoplasty is indicated for three or more levels of compression – shorter segment decompressions may not allow adequate “drift back”.32
Figure 3 depicts motion preservation with laminectomy and laminoplasty. [Figure 3]
Recent Controversies
Recently, Degenerative Cervical Myelopathy (DCM) has emerged as an umbrella term for many age-related and genetically associated pathologies.33,34 DCM includes cervical spondylotic myelopathy, degenerative disc disease, OPLL, and other conditions.33 The similarity between the three aforementioned conditions is that they all produce chronic spinal cord compression; common symptoms include decreased hand dexterity, gait imbalance, and genitourinary or sensorimotor disturbances.33 The similarities between DCM conditions result in an ongoing debate regarding clinical decision-making, ideal intervention timing, and best surgical approach.33 Out of the need for advancements in the management of DCM emerged an international multistakeholder consensus group called Research Objectives and Common Data Elements RECODE-DCM.34 Notably, RECODE-DCM has refined the definition of DCM.
CONCLUSION
CM patients present with various clinical manifestations along the spectrum ranging from mild to severe. Onset is often insidious, so early accurate diagnosis may be challenging. MRI remains the gold standard for confirmatory imaging after a thorough history and physical. Various surgical techniques and approaches exist to address cervical stenosis, each with pros and cons. Essential to any successful surgical treatment is timely diagnosis and adequate decompression of the cord to halt the progression of symptoms when indicated in moderate to severe cases.
Declaration of conflict of interest
Dr. Andrew Sama has an array of interests that present potential conflicts. He holds an ownership interest in Centinel Spine via Vbros Venture Partners V. He also serves as a consultant and member of the Scientific Advisory Board for Clariance, Inc. Dr. Sama holds similar roles as an advisor and consultant for Depuy Synthes Products, Inc., a Johnson & Johnson Company. He also has ownership interests in HSS ASC Development Network, LLC, HS2, LLC, ISPH 3 Holdings, LLC, and ISPH II, LLC. He lends his expertise as a consultant and serves on the Strategic Advisory Board for Kuros Biosciences. At Ortho Development Corp., he takes on multiple roles, working as a consultant, designer, and also receiving royalties. He receives research support from Spinal Kinetics. Furthermore, Dr. Sama also has an ownership interest in VBros Venture Partners X and Vestia Ventures MiRus Investment, LLC.
Dr. Frank Cammisa holds various roles that represent potential conflicts of interest. He is affiliated with 4WEB Medical as an Advisory Board member, consultant, and has ownership interest, in addition to receiving research support. He serves as a consultant for Accelus and receives royalties, as well as for Synexis, LLC, and NuVasive, Inc., with the latter also providing him with research support and royalties. He also receives research support from Camber Spine and Centinel Spine, Inc., formerly known as Cell Medica. HealthPoint Capital Partners, LP, ISPH II, LLC, ISPH 3 Holdings, LLC, related to Integrity Implants, Ivy Healthcare Capital Partners LLC, Orthobond Corporation, VBVP VI, LLC, which is a partnership in Centinel, and VBVP X, LLC, formerly Centinel Spine, Inc., are entities where he holds ownership interests. Furthermore, he is a part of the Scientific Advisory Board at HealthPoint Capital Partners, LP and the Advisory Board at Orthobond Corporation and Woven Orthopedic Technologies. He serves as a consultant and has an ownership interest at Spine Biopharma, LLC. He also has ownership interests at Medical Device Partners III, LLC’s Expanding Innovations and Tissue Differentiation Intelligence, LLC.
Dr. Federico Girardi has a series of potential conflicts of interest. These include ownership interests in Alphatec Holdings LLC, Bonovo Orthopedics, Inc., Centinel Spine, Paradigm Spine, LLC, and Tissue Differentiation Intelligence. His advisory roles are with Centinel Spine, EIT Emerging Implant Technologies, Healthpoint Capital Partners, LP, Paradigm Spine, LLC, Spinal Kinetics, Inc., and Spineart USA. Furthermore, he has acted as a consultant for DePuy Synthes Spine, EIT Emerging Implant Technologies, Ethicon, LANX, Inc., NuVasive, Inc., and Ortho Development Corp., in addition to his roles as a designer for DePuy Synthes Spine, LANX, Inc., NuVasive, Inc., and Ortho Development Corp. He also receives royalties from DePuy Synthes Spine for the development of the Lateral Access and Implant System, from LANX, Inc. for the Next Generation Spinous Process Fixation System, from NuVasive, Inc. for the development of Osteoporotic Fixation Implants, and from Ortho Development Corp. for the design and consultation services for Pagoda Spine System. In addition, he has an investment interest in Healthpoint Capital Partners, LP and has received research support from MiMedx Group, Inc. Lastly, he holds stock options in Spinal Kinetics, Inc.
Declaration of funding
The authors received NO financial support for the research, authorship, and publication of this manuscript.
Declaration of ethical approval for study
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Institutional review board approval was waived.
Declaration of informed consent
There is no information in the submitted manuscript that can be used to identify any patients.
Acknowledgments
Not applicable.